COMUNICACIONES
S. A. SICKLES1 PhD, J. KRUZE2 M.V., PhD, R. N. GONZALEZ1 DVM, PhD.
1 Quality Milk Promotion Services,
College of Veterinary Medicine, Cornell University, New York 14850, U.S.A.;
2 Mastitis Laboratory, Microbiology
Department, Faculty of Sciences, Universidad Austral de Chile, P.O. Box 167,
Valdivia, Chile.
SUMMARY
During July 1997, 71 bulk tank milk samples from dairy herds located in southern Chile were examined for mycoplasma by bacteriological culture. Fifty-three composite milk samples from cows with clinical mastitis or high somatic cell counts were also examined. Isolates were differentiated to species level by an indirect immunoperoxidase test. Mycoplasma bovis was isolated from five herds while Acholeplasma laidlawii was isolated from two herds. Only one cow milk sample yielded A.laidlawii; the remaining 52 samples were negative for mycoplasma. This is the first report of isolation of mycoplasma from cow milk in Chile. Veterinarians and dairy farmers should be aware that mycoplasma bovine mastitis, a highly contagious disease, is present in dairy herds in Chile.
Palabras Claves: bovino, mastitis, Mycoplasma.
Key words: bovine, mastitis, Mycoplasma.
INTRODUCTION
Mycoplasmas have been associated with a wide range of infectious diseases of cattle, e.g. pneumonia, arthritis, synovitis and mastitis (Hjerpe and Knight, 1972; Langsford, 1977; González et al., 1993). Dairy cattle of different ages may be asymptomatic carriers of mycoplasmas and several species of these microorganisms are part of the natural flora of the respiratory and urogenital tracts of these animals.
Mycoplasma bovine mastitis was first reported in England in 1960 (Davidson and Stuart, 1960; Stuart et al., 1963), Mycoplasma bovigenitalium being the etiologic agent. The first outbreak of the disease caused by M.bovis was reported in Connecticut, USA, in 1961 (Hale et al., 1962). Later a number of epizootic outbreaks of M.bovis mastitis were reported in the state of New York (Carmichael et al., 1963). Since then mycoplasma mastitis has been diagnosed in many parts of the world (Jasper, 1981), but not in Chile. This type of mastitis could be a highly devastating disease for a dairy herd because: 1) it is a contagious disease; 2) antibiotic treatment is ineffective; 3) clinical signs persist for variable periods of time (Boughton, 1979; Jasper, 1979; Bushnell, 1984; González and Sears, 1994); and, 4) cows with chronic subclinical mastitis frequently spread the infection to other cows and may carry the organism for several months or years (Jasper, 1979; González et al., 1993; González and Sears, 1994).
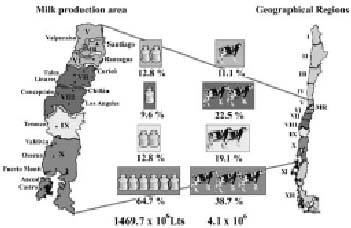
Milk production in Chile has increased during the last twelve years at a rate of approximately 10% yearly, reaching a historic record of 1530 million liters of milk delivered to dairy industries in 1998; however, a reduction of 4.2% of total milk reception in dairy industries was observed in 1999 (Chile, 2000). Although dairy cows are raised all over the country the milk production area is between the Vth Region (north) and the Xth Region (south), dairy farming being one of the most important activities in southern regions (IXth and Xth) where about 80% of total milk is produced (fig.1).
The national milk production average is less than 3000 liters per lactation but many dairy farms have an average yield between 7000 and 9000 liters/cow/lactation. Over 90% of dairy cows are machine milked and most dairy farmers practice post milking teat disinfection and dry cow therapy as routine mastitis control measures.
Bovine mastitis is a common disease among dairy cows in southern Chile and, although most cows are housed during winter, the major mastitis pathogen more frequently isolated from both clinical and subclinical mastitic cows is Staph.aureus (Zurita, 1988; Kruze, 1992; Chávarry, 1994; León, 1997). One of the authors* has observed that some large dairy farms in southern Chile have been suffering of a high incidence of clinical mastitis refractory to antibiotic treatment, with low bulk milk somatic cell count, and negative results on conventional culture media. However no attempt has been previously done to isolate mastitis pathogens other than the traditional ones.
The present communication reports the isolation and differentiation of mycoplasma species from bulk tank milk samples and from individual cows from dairy herds in southern Chile.
Figure 1. Milk production and cattle population of geographical regions in Chile (1999).

MATERIAL AND METHODS
Dairy herds. All dairy herds involved in this study were enrolled in the Genetic Improvement Program of COOPRINSEM, a private milk producers'cooperative located in Osorno (Xth Region), which are monthly sampled for milk production and quality records. The average herd size ranged between 80 and 800 milking cows with seasonal calving in autumn and spring and winter housing in individual stalls. Cows are fed concentrate food and silage during the winter months and concentrates plus grazing the remaining of the year. All cows were machine milked twice a day and the milk cooled in refrigerated bulk tanks until collected by the milk plant. A total of 71 dairy herds were included of which 64 were located in the Xth Region, 5 in the IXth Region and 1 each in the Vth and VIIIth Regions. Dairy herds in the Xth Region were well distributed all around the Region, the most important milk producing area in the country.
Bulk tank milk samples. During July 1997 convenience bulk tank milk samples from all herds submitted to the Milk Quality Laboratory of Cooprinsem (Osorno, Xth Region) were bacteriologically examined for mycoplasma. Milk samples were collected by trained personnel of COOPRINSEM, submitted, received, and stored in frozen conditions until examined for mycoplasma (within 7 days).
Cow milk samples. In mid July, 53 cow milk samples were aseptically collected by veterinary practitioners from two dairy herds in the Xth Region. All samples were submitted and stored at 4-7ºC until cultured for mycoplasma (Mastitis Laboratory, Microbiology Department, Universidad Austral de Chile, Valdivia), no later than 20 h after collection.
In the first herd, composite milk samples were collected from 15 cows with a history of decreased milk production and clinical mastitis. Some of these cows had more than one quarter affected at the time of sampling, some exhibited chronic mastitis, and other had no response to different antibiotic therapies following laboratory antibiograma tests.
In the second herd, composite milk samples were collected from 38 cows, 5 with high somatic cell counts and 33 with clinical mastitis. In this herd M. bovis had been previously detected at the beginning of July during the examination of bulk tank milk samples.
Bacteriological diagnosis. Bulk tank and cow milk samples were inoculated on a modified Hayflick medium (Freundt, 1983), following the procedure described elsewhere (González et al., 1992). Bulk tank samples were thawed, homogenized, and 0.05 ml spread onto the surface of half a standard petri dish using a sterile cotton swab. Inoculation of plates with cow milk samples were done by dipping cotton swabs into the milk sample and then spreading 0.02 ml onto a quarter of a plate of Hayflick medium. All plates were placed in a candle jar in an aerobic incubator at 37ºC and examined for mycoplasma growth every other day for ten days. If after ten days of incubation there were no visible mycoplasma colonies the sample was recorded as negative.
Isolation of bacterial L-forms was checked by five consecutive passages of colonies in modified Hayflick broth enriched with horse serum but without antibiotics and subsequent culture on blood agar plates. Differentiation of mycoplasma isolates was determined by an indirect immunoperoxidase test (Imada et al., 1987). Blank sensitivity discs soaked in rabbit antisera against M. bovis, M. bovigenitalium, M. canadense and A. laidlawii (Quality Milk Promotion Services, Cornell University, Ithaca, NY, USA) were used for species differentiation. Goat anti-rabbit immunoglobulin G horseradish peroxidase (Sigma Laboratories, St. Louis, MO, USA) was used as a conjugate in the test.
RESULTS AND DISCUSSION
Mycoplasma bovis was recovered from 5 (7%) and A. laidlawii from 2 (2.8%) of the 71 bulk tank milk samples cultured. All these M. bovis-positive samples were from herds located in the Xth Region.
A. laidlawii was isolated from 1 (1.9%) of the 53 cow composite milk samples examined, and it was recovered from a cow in herd Nr 2. Bacterial L-forms were never isolated from either bulk tank milk nor cow milk samples.
This is the first report of mycoplasma isolation from cow milk in Chile. The procedure used, i.g. bacteriological examination of bulk tank milk samples, is useful for detection of mycoplasma in dairy herds as well as for epidemiological surveillance and monitoring of mycoplasma mastitis (González et al., 1992). The presence of mycoplasma in bulk tank milk is the result of shedding from infected udders (Jasper, 1980; González et al., 1986; González et al., 1988). Therefore, its isolation from five bulk tank milk samples implies that M. bovis-infected cows existed in those herds at the time of sampling. However, a negative mycoplasma culture from a bulk tank does not guarantee the absence of mycoplasma mastitis in a herd (Jasper et al., 1966; González and Sears, 1994).
All the pathogenic isolates recovered in this study were M. bovis. This is the most common as well as the most pathogenic mycoplasma causing bovine mastitis (Gourlay and Howard, 1979; Jasper, 1982; Bushnell, 1984; González et al., 1992). Mycoplasma mastitis may be clinical, subclinical or chronic. Cows with infected udders may continue to shed mycoplasma and suffer from decreased production in subsequent lactations or may spontaneously self cure. Milk yield in subsequent lactations may be lower, equal or higher than expected (González and Sears, 1994). Carrier cows are a source of infection to other cows in the herd. A recent study in the states of New York and Pennsylvania showed that of all mastitis pathogens analyzed economic loss per case per lactation was highest for mastitis caused by mycoplasma (Wilson et al., 1997).
Acholeplasma laidlawii was also isolated from one cow and two bulk tank milk samples. This microorganism by itself is not considered pathogenic to cattle, although it has been isolated from mastitic milk (Gourlay and Howard, 1979). Bushnell (1984) considered this agent as a common contaminant of the cow's environment.
Furthermore, California researchers found that many cow and bulk tank milk samples were contaminated with A. laidlawii during rainy months (Jasper, 1981). Therefore, and particularly when bulk tank milk samples are to be examined, identification of Mycoplasma-like organisms is recommended (Bushnell, 1984).Bacterial L-forms can sometimes be recovered from cow milk as a consequence of prolonged antibiotic treatments (Sears et al., 1987). As antibiotic treatment is a practice widely used for mastitis therapy in southern Chile, all isolates were checked for bacterial L-forms to avoid misidentification. However, misdiagnosis with Mycoplasma is improbable (Jasper, 1981).
The isolation of M. bovis from bulk tank milk from dairy herds in Chile implies that: 1) the organism exists as an agent of contagious mastitis; 2) the disease can be spread to uninfected herds through the sale of infected animals; 3) uninformed people, such as veterinarians, milk plant truck drivers, milking equipment dealers, family members and friends, can transmit the disease from infected to uninfected dairies (Bushnell, 1984; González et al., 1992); 4) severe disease outbreaks could occur if replacements are commingled with existing herds without quarantine and bacteriological testing (González et al., 1995; Wilson and González, 1997).
RESUMEN
Durante el mes de julio de 1997 se realizaron exámenes bacteriológicos para el aislamiento de micoplasmas en 71 muestras de leche de estanque provenientes de rebaños lecheros del sur de Chile. Además, se cultivaron 53 muestras compuestas de leche de vacas con mastitis clínica o con elevado recuento de células somáticas. Los aislamientos de Mycoplasma se identificaron a nivel de especie mediante una prueba indirecta de inmunoperoxidasa. Mycoplasma bovis se aisló en 5 rebaños y Acholeplasma laidlawii en dos rebaños. De las muestras individuales de vaca sólo uno resultó positiva a A. laidlawii, siendo las 52 restantes negativas a Mycoplasma. Esta es la primera comunicación sobre aislamiento de micoplasmas de leche de vacas en Chile. Los médicos veterinarios y productores lecheros deberían tener presente que la mastitis por micoplasma, una enfermedad altamente contagiosa del bovino, está presente en los rebaños lecheros del sur de Chile.
ACKNOWLEDGMENTS
The authors wish to thank Dr G. Stolzenbach and Dr F.Santibáñez from COOPRINSEM Osorno, for allowing the use of laboratory facilities in Osorno and Dr B.León for her valuable technical assistance in collection and processing of milk samples at the Milk Quality Laboratory, COOPRINSEM, Osorno.
* J. Kruze, personal communication.
_________________________________________
Aceptado: 01.08.2000.
REFERENCES
BOUGHTON, E. 1979. Mycoplasma bovis mastitis. Vet. Bull. 49: 377.
BUSHNELL, R.B., 1984. Mycoplasma mastitis. Vet. Clin. North Am. (Large Anim. Pract) 6: 301-312.
CARMICHAEL, L.E., R.S. GUTHRIE, M.G. FINCHER, L.E. FIELD, S.D. JOHNSON, W.E. LINQUIST, 1963. Bovine Mycoplasma mastitis. In:. Proc. 67th Ann. Mtg U.S. Livestock Sanitary Ass., pp. 220-234.
CHAVARRY, M., 1994. Terapia de secado. Efecto del método de administración del antibiótico sobre la eficiencia del tratamiento y la presencia de neoinfecciones intramamarias durante el período seco en vacas lecheras. M.Sc. Thesis, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile, 104 pp.
CHILE, 2000. Boletín de la leche 1999. Oficina de Estudios y Políticas Agrarias (ODEPA), Departamento de Información Agraria, Ministerio de Agricultura, Chile. 51 pp.
DAVIDSON, I., P. STUART, 1960. Isolation of Mycoplasma-like organism from an outbreak of bovine mastitis (letter). Vet. Rec. 72, 706.
FREUNDT, E. A., 1983. Culture media for classic mycoplasmas. En: S. RAZIN and J.G. TULLY (eds.). Methods in Mycoplasmology, Vol. 1. Mycoplasma characterization. Academic Press, New York.
GONZALEZ, R.N., P.M. SEARS, 1994. Diagnosis, control, and effect on milk production of Mycoplasma bovis intramammary infections. En: Proc. XVIII World Buiatrics Cong., Bologna, Italy. pp. 681-684.
GONZALEZ, R.N., P.M. SEARS, D.J. WILSON, 1995. Epidemiology of mycoplasmal bovine mastitis in the state of New York, USA. En: Proc. III IDF Inter. Mastitis Seminar, Tel Aviv, Israel. pp. 68-69.
GONZALEZ, R.N., D.E. JASPER, R.B. BUSHNELL, T.B. FARVER, 1986. Relationship between mastitis pathogen numbers in bulk tank milk and bovine udder infections in California dairy herds. J. Am. Vet. Med. Ass. 189: 442-445.
GONZALEZ, R.N., B.M. JAVARAO, S.P. OLIVER, P.M. SEARS, 1993. Pneumomia, arthritis and mastitis in dairy cows due to Mycoplasma bovis. En: Proc. 32nd Ann. Mtg. Natl. Mastitis Counc., Kansas City, USA., pp. 178-186.
GONZALEZ, R.N., P.M. SEARS, R.A. MERRILL, G.L. HAYES, 1992. Mastitis due to Mycoplasma in the state of New York during the period 1972-1990. Cornell Vet. 82: 29-40.
GONZALEZ, R.N., D.E. JASPER, T.B. FARVER, R.B. BUSHNELL, C.E. FRANTI, 1988. Prevalence of udder infections and mastitis in 50 California dairy herds. J. Am. Vet. Med. Ass. 193: 323-328.
GOURLAY, R.N., C.J. HOWARD, 1979. En: J.G. TULLY, R.F. WHITCOMBS (eds). The Mycoplasmas, Vol. 2. Academic Press, New York.
HALE, H.H., C.F. HELMBOLDT, W.N. PLASTRIDGE, E.F. STULA, 1962. Bovine mastitis caused by a Mycoplasma species. Cornell Vet. 52: 582-591.
HJERPE, C.A., H.D. KNIGHT, 1972. Polyarthritis and synovitis associated with Mycoplasma bovimastitidis in feedlot cattle. J. Am. Vet. Med. Ass. 160: 1414-1418.
IMADA, Y., I. UCHIDA, K. HASHIMOTO, 1987. Rapid identification of mycoplasmas by indirect immunoperoxidase testing using small square filter paper. J. Clin. Microbiol. 25: 17-21.
JASPER, D.E., 1979. Bovine mycoplasmal mastitis. J. Am. Vet. Med. Ass. 175: 1072-1074.
JASPER, D.E., 1980. Prevalence of mycoplasmal mastitis in the western states. Calif. Vet. 43: 24-26.
JASPER, D.E., 1981. Bovine mycoplasmal mastitis. En: C.E. CORNELIUS and B.F. SIMPSON (eds). Advances in Veterinary Sciences and Comparative Medicine, Academic Press, New York.
JASPER, D.E., 1982. The role of mycoplasma in bovine mastitis. J. Am. Vet. Med. Ass. 181: 158-162.
JASPER, D.E., N.C. JAIN, L.H. BRAZIL, 1966. Clinical and laboratory observations on bovine mastitis due to mycoplasma. J. Am. Vet. Med. Ass. 148: 117-129.
KRUZE, J., 1992. Etiología y epidemiología de la mastitis. Rev. Holstein Chile 34: 14-15.
LANGSFORD, E.U., 1977. Mycoplasma agalactiae sub.sp. bovis in pneumonia and arthritis of bovine. Can. J. Comp. Med. 41: 89-94.
LEON, B., 1997. Frecuencia de aislamiento de los principales agentes de mastitis en el sur de Chile. Rev. Cooprinforma 40: 1-6.
SEARS, P.M., M. FETTINGER, J. MARSH-SALIN, 1987. Isolation of L-form variants after antibiotic treatment in Staphylococcus aureus bovine mastitis. J. Am. Vet. Med. Ass. 191: 681-684.
STUART, P., I. DAVIDSON, G. SLAVIN, F.A. EDGSON, D. HOWELL, 1963. Bovine mastitis caused by a mycoplasma. Vet Rec. 75: 59-64.
WILSON, D.J., R.N. GONZALEZ, 1997. Evaluation of milk culture, SCC and CMT for screening herd additions. En: Proc. 36th Ann. Mtg. Natl. Mastitis Counc., Alburquerque, U.S.A. pp. 127-131.
WILSON, D.J., R.N. GONZALEZ, H.H. DAS, 1997. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J. Dairy Sci. 80: 2592-2598.
ZURITA, L., 1988. Mastitis bovina: situación nacional. Patol. Anim. 2: 36-41.