REVISION BIBLIOGRAFICA
The oviductal sperm reservoir in domestic mammals
Reservorio de espermatozoides en el oviducto de mamíferos domésticos
P Bosch*, R W Wright Jr.
Department of Animal Sciences, Washington State University
SUMMARY
In all mammalian species studied to date, sperm cells are arrested in the caudal isthmus before ovulation to form an oviductal reservoir. When ovulation occurs, discrete numbers of sperm are released from this region and ascend to the ampulla where fertilization normally occurs. Experimental evidence indicates that the sperm reservoir is instrumental in delivering the appropriate number of sperm in the correct physiological state for normal fertilization. In this paper, we present a comprehensive review of the sperm reservoir formation and functions in mammals. Sperm reservoir manipulation as a potential tool to improve reproductive performance is also discussed.
Key words: sperm reservoir, sperm transport, oviduct, domestic animals.
Palabras clave: reservorio de espermatozoides, transporte de espermatozoides, oviducto, mamíferos domésticos.
RESUMEN
En todas las especies estudiadas hasta el presente, los espermatozoides se acumulan en la porción caudal del istmo antes de la ovulación, formando un reservorio de espermatozoides en el oviducto. Luego de la ovulación un número preciso de espermatozoides es liberado desde esta área y ascienden hacia la ampolla del oviducto donde ocurre la fertilización. La evidencia experimental indica que el reservorio de espermatozoides es importante para proveer un número apropiado de espermatozoides en condiciones fisiológicas óptimas para fertilizar el ovocito. En este artículo presentamos una revisión exhaustiva de las características y funciones del reservorio oviductal de espermatozoides en mamíferos, como así también su manipulación como una herramienta potencial para mejorar la eficiencia reproductiva.
INTRODUCTION
It is clear that the oviduct plays a central role in successful reproduction in domestic mammals. The oviduct not only participates in ovum and sperm transport to the site of fertilization, but also provides the microenvironment where the process of conception and early embryonic development occur. Studies in vivo and in vitro (Chian and Sirard, 1995; Pacey et al 1995b, Pollard et al 1991, Suárez 1998, Suárez et al 1997, Thomas and Ball 1996, Thomas et al 1994b) indicate that sperm cells accumulate in the isthmus around the time of ovulation, constituting an oviductal sperm reservoir. After ovulation, sperm cells are gradually released from this region and reach the site of fertilization to meet the female gamete. A low sperm number at the site of fertilization is a constant finding in domestic species (sperm:oocyte ratio close to 1) (reviewed in Hunter, 1996); this phenomenon reduces the risk of polyspermy, a pathological condition that invariably leads to developmental failure of the zygote in eutherian mammals.
Since oocyte maturation, fertilization and embryo development can be achieved completely by in vitro procedures, the oviduct might be considered a non-essential organ. In addition, pregnancies have been reported in animals with oviductal dysfunctions (e.g., lack of oviductal cilia). However, in the context of animal production, successful reproduction must be considered in terms of a population rather than an individual basis; from this perspective, the oviduct and its functions are essential to achieve high reproductive performance.
ANATOMICAL BASES OF SPERM RESERVOIR
The mammalian oviduct can be divided into three different anatomical regions: infundibulum, ampulla, and isthmus, each associated with distinct physiological functions. The fimbriated infundibulum, the rostral portion of the oviduct, is responsible for oocyte transport into the tube after ovulation. The ampulla represents a dilated tubular region in which the process of fertilization is completed. Finally, the isthmus is involved in gamete and embryo transport and considered the anatomical base of the sperm reservoir (Hunter 1984, Suárez 1987). The oviductal wall is comprised of three distinctive layers: an external serosal mesosalpinx, an intermediate double-layered miosalpinx and an internal endosalpinx. The latter, also called oviductal mucosa, consists of one layer of columnar epithelial cells. This epithelium contains ciliated (most cells) and non-ciliated (secretory) cells. The oviductal mucosa is arranged in folds which increase in complexity from the utero-tubal junction to the ostium.
Using a surgical approach of oviductal ligation and transection at different times after mating, Hunter (1984) and Willmut et al (1984) have demonstrated that the caudal isthmus and utero-tubal junction have the function of sperm storage in pigs and cattle. In the horse, the isthmus may also function as a site for sperm storage, since more sperm were found attached to explants from the isthmus than those from the ampullary region (Thomas et al 1994a). Suárez (1987) observed more sperm in the isthmus compared with those present in the ampulla of excised mouse oviducts. The physical obstruction caused by the narrowness of isthmic lumen along with deep furrows formed by complex folds of the mucosa may contribute to sperm entrapment in this region (Hunter 1995). In addition, these features might enhance the probability of association between specific molecules present on sperm and oviductal cells, and therefore increase the number of sperm attached to oviductal epithelial cells (OEC).
SPERM RESERVOIR FORMATION
Factors involved in sperm reservoir formation. Since Suárez and colleagues first defined the ligand-receptor-like nature of sperm-OEC attachment (Demott et al 1995, Lefebvre et al 1997, Suárez 1998, Suárez et al 1998), it has been hypothesized that direct sperm-OEC contact is the major mechanism underlying sperm reservoir formation (Suárez et al 1990). This hypothesis is further supported by studies in which sperm remained attached even after energetic washing (Smith and Yanagimachi 1990) or proteolytic enzyme treatment (Raychoudhury and Suárez 1991). Although this could be true, the relative contribution of each factor in the oviductal reservoir formation remains unclear. Mucus secretions, chemical properties of oviductal fluid, temperature gradients, patency of the lumen, and sperm binding to OEC can all contribute, to different degrees, to establishment of the sperm reservoir.
Biochemical nature of oviduct-spermatozoon interaction. Oviduct-sperm binding is a reversible process that appears to involve oligosaccharide moieties on the epithelial cell membrane (Demott et al 1995, Dobrinski et al 1996a, Suárez 1998, Suárez et al 1998) and a Ca2+-dependent lectin on the sperm surface (Suárez et al 1998). In all species studied to date, different sugars have been demonstrated to participate in this phenomenon (Demott et al 1995, Dobrinski et al 1996a, Lefebvre et al 1997, Suárez et al 1998). In hamsters, sialic acid and fetuin (a sialoglycoprotein) reduced the number of sperm adherent to oviductal explants suggesting that sperm-oviductal interaction is mediated, at least in part, by these molecules (Demott et al 1995). Molecules involved in equine sperm-OEC interaction in vitro have also been investigated by Dobrinski et al (1996a). In this study, addition of galactose or glycoproteins with exposed galactosyl residues to equine sperm-OEC cocultures inhibited sperm attachment to the cells. In pigs, formation of the sperm reservoir appears to involve the interaction of mannosyl-oligosaccharide ligands on the oviductal cells with binding molecules on the sperm surface (Topfer-Petersen et al 2002). Experimental data indicate that fucose is the particular carbohydrate moiety that constitutes the binding site on bovine oviduct epithelia (Lefebvre et al 1997, Suárez et al 1998) (figure 1). Further characterization of this interaction (Suárez et al 1998), established that a Lewis-a trisaccharide-like molecule is involved in sperm-oviductal epithelial cell binding. This oligosaccharide, which has a fucose molecule in its composition, had greater ability to inhibit sperm attachment to OEC than fucose alone (Suárez et al 1998).
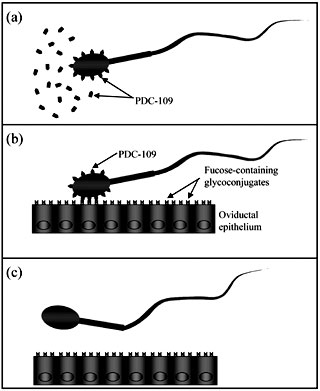 |
| Figure 1. Proposed model to
explain bull sperm attachment to and release from oviductal epithelium
(adapted from Suárez, 2002). (a) PDC-109, a protein secreted by the
seminal vesicles, associates with sperm's plasma membrane. Excess
PDC-109 present in seminal fluid may preclude sperm binding to lower
portions of the female reproductive track. (b) In the oviduct, spermatozoa
PDC-109 recognizes fucosylated residues present on epithelial cells
contributing to sperm entrapment in the lower portion of the oviduct
to form a sperm reservoir. (c) Lose or modification of PDC-109 from
spermatozoa surface associated with capacitation facilitates sperm
release from the reservoir.
Modelo propuesto para explica la asociación y posterior liberación de los espermatozoides bovinos desde el epitelio oviductal (adaptado de Suárez, 2002). (a) PDC-109, una proteína secretada por las vesículas seminales, se asocia a la membrana plasmática de los espermatozoides. La presencia de PDC-109 libre en el plasma seminal impediría la unión de los espermatozoides a porciones posteriores del tracto reproductor de la hembra. (b) En el oviducto, el PDC-109 ligado a la superficie de los espermatozoides reconoce residuos fucosilados presentes en las células del oviducto. Esta interacción permite la adherencia de los espermatozoides al epitelio contribuyendo de este modo a la formación del reservorio. (c) Pérdida o modificaciones de la proteína PDC-109 como consecuencia de la capacitación espermática facilita la liberación de los espermatozoides del reservorio. |
Structural and functional evidence indicate that the fucose-binding lectin present on bull sperm is a seminal plasma protein secreted by the seminal vesicles called PDC-109 (BSP-A1/A2) (Gwathmey et al 2003, Ignotz et al 2001). Upon ejaculation, this protein associates with the sperm's plasmatic membrane enabling it to bind to the oviductal epithelium (figure 1). This proposed mechanism explains the limited binding capacity of sperm that have not been in contact with seminal fluids i.e., epididimal sperm (Gwathmey et al 2003, Petrunkina et al 2001). Furthermore, excess PDC-109 in seminal plasma may block sperm binding sites on posterior portions of the female reproductive tract facilitating sperm transport to the oviducts (Suárez, 2002).
Mucus secretions. Overstreet and Cooper (1975) first observed that rabbit sperm collected from the isthmus of does before ovulation were immotile or weakly motile. This observation is interpreted as a transitory inhibition or suppression of sperm flagellar movement, since motility could be restored by dilution of immotile sperm with medium or ampullar fluid (Overstreet et al 1980). During sperm storage in the cauda epididymides, a proteinaceous secretion that increases the viscosity of epididymal fluid inhibits sperm flagellar movement. A mucus secretion has been described in cattle (McDaniel et al 1968), rabbits (Jansen and Bajpai 1982), pigs (Hunter 1995) and the human isthmus (Jansen 1978). This secretion may suppress sperm motility in an analogous manner to viscous epididymal secretions. Suárez and coworkers have observed sperm entrapped in a mucus-like secretion produced in vitro by oviductal explants from cows (Suárez et al 1990) and gilts (Raychoudhury and Suárez 1991). However, in these studies the motility of sperm entrapped in mucus was not thoroughly characterized. In scanning microscopy microphotographs, the oviductal mucus can be seen distributed on the sperm head forming relatively large spheres or granules (Hunter et al 1991). Suárez et al (1998) observed the narrow isthmic lumen filled with mucus secretions in histological sections of bovine oviducts fixed by rapid freezing to preserve the tissue architecture. Similarly, intraluminal fluid in preovulatory porcine oviducts contains abundant glycosaminoglycans which obliterate the narrow lumen of the sperm reservoir segment (Rodríguez-Martínez et al 2001). Hyaluronan is one of the mucopolysaccharides produced by the isthmic epithelium which reaches maximum concentration in the luminal fluid during estrus (Rodríguez-Martínez et al 2001). This observation in conjunction with the known ability of hyaluronan to modulate sperm capacitation in vitro (Suzuki et al 2000, Suzuki et al 2002), has led to the hypothesis that mucopolysaccharides may have a role in oviductal sperm transport (Rodríguez-Martínez et al 2001). It is probable that narrowness of the isthmus in conjunction with mucus secretions present during the preovulatory stage can impede or retard sperm transit, contributing to sperm reservoir formation (Suárez et al 1997).
Chemical properties of oviductal fluid. The oviductal fluid represents the aqueous milieu in which female and male gametes and embryos are suspended during oviductal transit. This fluid is a complex mixture of ions and macromolecules solublilized in water. Some constituents are derived from the plasma, while others are synthesized in the epithelia and secreted into the lumen (Leese, 1988). Since the ionic composition of oviductal fluid is different from that of plasma, it is assumed that a differential or selective ion transport is present in the oviductal wall. For example, oviductal fluid composition is characterized by a high potassium and low calcium concentration compared to plasma levels of these ions (Borland et al 1977). Studies in the rabbit indicate that low concentrations of potassium can inhibit sperm motility, a suppression that can be reversed by pyruvate (Burkman et al 1984). Data of Murdoch and White (1971) suggest that potassium inhibition of sperm motility is mediated by reduction of oxygen consumption and glycolysis. Differences in oviductal fluid composition during the estrous cycle (Jansen, 1984), and between ampulla and isthmus regions have been reported (Nichol et al 1992). In pigs, glucose and lactate have been shown to be present in isthmic fluid at different concentrations from those in ampullar fluid (Nichol et al 1992). These regional differences in ionic and macromolecular composition of oviductal fluid can promote preovulatory sperm arrest in the caudal oviduct.Temperature gradients. Another factor that may be involved in sperm storage in the oviduct is a temperature gradient along this organ. Hunter and Nichol (1986) have measured the temperature along the oviduct of sows before and after ovulation. They found that the isthmus was 0.75°C cooler than the ampulla during the preovulatory stage, but this difference was no longer detected after ovulation. David et al (1971) have reported similar regional temperature differences in the rabbit oviduct. These temperature variations may reflect differences in regional vascular and lymphatic flux that in turn can affect the oviductal fluid composition. Increased contractility of the myosalpinx in the ampullar region during the preovulatory period may also be responsible for temperature gradients. Although of low magnitude, these temperature differences between ampulla and isthmus may directly or indirectly inhibit sperm motility and contribute to sperm reservoir formation.
Patency of the oviductal lumen. The mucosal surface of the isthmus is arranged in complex folds that reduce the patency of the organ. A distinctive characteristic of the isthmus is a well-developed smooth muscle layer located between mucosa and serosa (El-Banna and Hafez 1970). The smooth muscle in the isthmic region is profusely innervated by cholinergic fibers from the sympathetic nervous system that control tonic muscle contraction (Hunter 1995). In addition, high numbers of adrenergic receptors are present on the cells of the circular smooth muscle layer in the isthmus (Brundin 1965); stimulation of these receptors can significantly reduce the oviductal lumen. High levels of estrogen during estrus can increase the tonic smooth muscle contraction (Hunter 1996), an effect that is probably mediated through adrenergic receptors mentioned above. Moreover, estrogen enhances oviductal obstruction by increasing the height of mucosal epithelium (McDaniel et al 1968) and by stimulating edema of the oviductal wall (Boyle et al 1987). All these factors, plus changes in direction and/or intensity of ciliar beating seem to be involved in the buildup of sperm in the caudal isthmus prior to ovulation.
FACTORS AFFECTING SPERM ENTRAPMENT AND RELEASE FROM THE SPERM RESERVOIR. After ovulation, sperm cells are gradually released from the reservoir in the caudal isthmus and ascend to the ampulla, ensuring a sperm/egg ratio close to 1 at the site of fertilization. Capacitating-like changes in sperm plasma membrane seem to be responsible for sperm release from oviductal epithelium (Revah et al 2000). Sperm exposed to capacitating conditions have limited ability to attach to oviduct cells in vitro (Lefebvre and Suárez 1996). Uncapacitated sperm initially bind to OEC both in vivo (Smith and Yanagimachi 1991) and in vitro (Fazeli et al 1999). This initial attachment is followed by induction of capacitation and release of capacitated sperm (Fazeli et al 1999; Smith and Yanagimachi 1991). We have demonstrated that heparin, a glycosaminoglycan known to induce bull sperm capacitation in vitro, is a fast and highly effective inductor of sperm release from OEC in vitro (Bosch et al 2001). The heparin-induced sperm release reported in our and another study (Talevi and Gualtieri 2001) may reflect change or loss of binding molecules on the sperm plasma membrane, which in turn promotes sperm release. Additionally, development of sperm hyperactive movement (Pacey et al 1995a) and the hormonal shift from follicular to luteal phase could participate in this process (Hunter 1995). Changes in the sperm membrane associated with capacitation, acquisition of sperm hypermotility and modification of the female endocrine status may all influence sperm-oviduct cell dynamics. However, the mechanisms that regulate timely sperm liberation from the site of storage after ovulation are not yet fully understood.
IS SPERM RELEASE FROM THE OVIDUCTAL RESERVOIR REGULATED? One of the most striking features of the vascular anatomy of the female reproductive tract in ruminants and swine species is the close apposition and extensive contact between ovarian and uterine vessels (Ginther 1976, 1974). Regulatory substances with low molecular weight such as hormones can pass from vein to artery and vice versa without the necessity of direct vascular connections; in this way, a bi-directional communication between ovary and uterus can be established. In the sheep, it has been shown that not only estrogen, progesterone and testosterone (McCracken et al 1984) but also oxytocin (Schramm et al 1986) is interchanged between arteries and veins in the ovarian pedicle. Furthermore, the local venoarterial pathway that follows prostaglandin F2a produced by the non-gravid uterus to cause luteolysis has been extensively demonstrated in both sheep and cattle (for review see: Ginther 1974).
Existence of a local counter-current transfer of gonadal hormones from the ovarian vein into the tubal branch of the ovarian artery has lead to the hypothesis of ovarian endocrine regulation of sperm progression to the site of fertilization (Hunter 1995, 1996). Through this mechanism, relatively high concentrations of ovarian hormones can reach the oviductal wall compared to systemic levels (Hunter 1995). Gonadal steroids could coordinate gamete transport to the site of fertilization by changing the composition and physical properties of oviductal fluid and mucus, gas tension, number of sperm binding sites and pattern of contractile activity (Hunter 1996). Since ovarian steroids can diffuse into the oviductal lumen, a direct effect of these hormones on sperm function and morphology cannot be excluded.
While estrogen can increase the number of spermatozoa bound to the oviductal epithelium, progesterone produces the opposite effect. The number of sperm attached to porcine oviduct explants was not different when oviducts were collected at estrus or midcycle (Suárez et al 1991). However, a significantly greater number of sperm were observed attached when estradiol had been added to the culture medium (Raychoudhury and Suárez 1991, Suárez et al 1991). Also in pigs, administration of progesterone as either local microinjections in the caudal portion of the isthmus (Hunter 1972) or systemically (Day and Polge 1968) increased the number of sperm released from the reservoir and, therefore, the incidence of polyspermia. In cattle, neither the female hormonal state nor the region of the oviduct seem to affect the number of sperm attached to oviductal explants in vitro (Lefebvre et al 1995) but the direct effect of gonadal steroids on sperm-oviduct interaction has not been thoroughly investigated (Suárez et al 1990).
FUNCTIONS OF THE SPERM RESERVOIR
SPERM CAPACITATION, ACROSOME REACTION AND MOTILITY. Capacitation is a complex phenomenon that involves profound modifications in sperm plasma membrane organization, structure and concentration of proteins, steroids and phospholipids (Yanagimachi 1990). Normally, capacitation is initiated after mating, once the sperm are in contact with the female reproductive tract.
However, the exact site where this process begins varies according to the site of semen deposition. In species such as the bovine in which the semen is deposited in the anterior vagina, capacitation begin during sperm migration through the cervix. In species with uterine ejaculation including pigs (Hunter 1980), the oviduct seems to be the major site of sperm capacitation. Although sperm capacitation is not site specific (e.g., uterine environment can fully support sperm capacitation), the primary organ where capacitation is completed is the oviduct. Studies in vitro have shown that sperm binding to oviductal cells (Chian et al 1995; Chian and Sirard, 1995) and soluble product(s) present in conditioned medium (Chian et al 1995) are both necessary for sperm capacitation. Parrish et al (1989) reported that the oviduct produces a potent capacitating factor that is present in the oviduct fluid. Interestingly, the highest activity of this factor was observed in oviductal fluid collected at estrus. Similarly, Chian et al (1995) reported that a capacitating factor was present in medium conditioned by OEC. The capacitating ability was maximal in medium conditioned by OEC collected one or two days after ovulation, or when estradiol-17b was added to the culture medium. In conjunction, these data suggest that sperm capacitation in the oviduct is influenced by hormonal changes associated with the estrous cycle. In addition to the capacitating factor, studies in human (Kervancioglu et al 1994), equine (Ellington et al 1993b), bovine (Chian and Sirard, 1995; Guyader and Chupin, 1991), ovine (Gutiérrez et al 1993) and porcine species (Fazeli et al 1999) have demonstrated that sperm contact with OEC is involved in the capacitation process. In the bovine species, Chian and Sirard (1995) reported a higher oocyte penetration rate when sperm cells were cocultured with OEC compared to those observed in the control group without cells.
The experimental evidence presented so far suggests that sperm-OEC contact and secretions from oviductal epithelia enhance sperm capacitation. Sperm capacitation is associated with reduction of life span of sperm cells (Hunter 1987). Then, if sperm-OEC interaction promotes capacitation, it is difficult to explain one of the major functions of the sperm reservoir which is to extend sperm fertilizing ability (Boquest and Summers 1999, Pollard et al 1991). As a consequence of modifications in sperm membrane composition, a concomitant influx of Ca2+ is observed during sperm capacitation (Dasgupta et al 1993). Dobrinski et al (1996), using a fluorescent calcium indicator, reported that sperm in contact with OEC maintained a low intracellular calcium concentration when compared to both free-swimming sperm and sperm in the control group without oviductal cells. Furthermore, it has been suggested that key pro-capacitation molecules like bicarbonate might be kept at lower levels in the sperm reservoir compared with other oviductal segments retarding in this way sperm capacitation (Rodríguez-Martínez et al 2001). According to this, sperm-OEC contact and the isthmic environment seem to retard rather than induce sperm capacitation, thus prolonging sperm fertilizing capacity.
A limitation to studying sperm physiology in coculture with OEC monolayers is that it is not possible to separate the effects of direct sperm-OEC contact from those induced by soluble products released by oviductal cells. To overcome this problem, Smith et al (1997) developed a method to isolate apical plasma membrane vesicles (AMV) from OEC. Coculture of sperm with AMV allows researchers to study the direct effects of sperm-OEC membrane contact on sperm function since cytoplasm is not present in the system. Using this approach, it has been shown that membrane contact between sperm and AMV can modulate intracellular calcium concentration, delay sperm capacitation, and extend sperm viability (Dobrinski et al 1997, Smith 1998, Smith and Nothnick 1997). Though the underlying mechanism(s) is(are) not currently understood, it is conceivable that intimate contact between sperm and OEC plasma membrane might stabilize the sperm membrane, thus retarding Ca2+ influx. Alternatively, recognition of specific molecules on OEC may trigger intracellular signals that lead to reduced permeability to Ca2+ and/or stimulate Ca2+ extrusion from the sperm.
Acrosome reaction consists of a series of morphological changes on the sperm head which ends when the sperm plasma membrane fuses with the outer acrosomal membrane and the enzymatic content from the acrosomal sac is released (Yanagimachi 1990). Capacitated sperm may undergo acrosome reaction if the appropriate stimuli are applied. It is classically assumed that the acrosome reaction in vivo starts when the spermatozoon passes through the cumulus cell layers or immediately after it contacts the zona pellucida. Bedford (1970) reported that two different kinds of acrosome reaction can occur: a) true acrosome reaction when an appropriate stimulus triggers a response in a previously capacitated sperm and b) false acrosome reaction represented by an unspecific loss of acrosomes in dying or dead sperm.
There does not seem to be a clear relationship between the occurrence of acrosome reaction and the presence of sperm in the oviductal sperm storage. However, most sperm cells attached to OEC both in vivo and in vitro have intact acrosomes (Ellington et al 1991, Gualtieri and Talevi 2000, Hunter et al 1991, Pollard et al 1991, Suárez et al 1991). In an electron microcopy study (Esponda and Moreno, 1998), 81% percent of adherent mouse sperm had intact acrosomes, a result that contrasted with the highly damaged acrosomes observed in the unattached sperm population.
In addition to electron microscopy studies, direct observation of sperm by light microscopy (Ellington et al., 1991), chlortetracycline stain (Ellington et al 1993a, Fazeli et al 1999), and a triple stain technique (Gutiérrez et al 1993) have been used to assess capacitation and acrosome status of sperm in coculture with OEC. A higher percentage of acrosome reacted sperm has been found in the unattached fraction compared to the bound sperm population (Ellington et al 1991, Gutiérrez et al 1993). This reflects the ability of OEC to selectively attach intact sperm cells and/or induce sperm capacitation. Thus, a proportion of released capacitated sperm may undergo false acrosome reaction and/or, what is called spontaneous acrosome reaction before they reach the site of fertilization.
Sperm transport in the female reproductive tract and penetration of the oocyte layers depend greatly on sperm motility. Furthermore, motility seems to be a precondition for sperm attachment to OEC since dead sperm do not interact with OEC (J. E. Ellington, personal communication). Development of reliable in vitro culture systems for oviductal cells (Joshi 1991, 1988, Walter 1995) has permitted the study of many aspects of sperm-OEC interactions in a system that mimics in vivo conditions. A few minutes after addition of a sperm suspension, actively motile sperm cells start to adhere to OEC explants (Lefebvre and Suárez 1996). Most sperm attach by their heads and have active tail movement (Chian and Sirard 1995), only a small proportion makes contact by the tail. Experimental data indicate that oviductal cells stimulate and extend sperm motility, not only through plasma membrane contact between sperm and OEC (Dobrinski et al 1997; Ellington et al 1998b, Kervancioglu et al 1994), but also through soluble products secreted by the cells (Abe et al 1995, Boquest et al 1999). Activation of sperm hypermotility, which is associated with sperm capacitation, occurs in sperm cocultured with OEC in vitro (Ellington et al 1993a, Ellington et al 1991; Lefebvre and Suárez 1996) and has been directly observed by transillumination in excised mouse oviduct shortly after mating (Suárez 1987). A change from progressive to hyperactivated motility may contribute to sperm release from the oviductal reservoir (Pacey et al 1995a).
A reduction or inhibition of sperm motility during the phase of sperm storage has been proposed as a mechanism to extend sperm viability and fertilizing capacity (Suárez et al 1990); however, objective studies involving motility patterns of sperm attached to OEC have not been reported. Further investigation of sperm metabolism during oviductal transport may clarify this controversial aspect.
SPERM SELECTION. Indirect evidence suggests that sperm attachment to oviductal cells in vivo is a prerequisite for fertilization, or at least that spermatozoa released from the reservoir are more likely to fertilize an oocyte. This hypothesis is supported by a study in which only a subpopulation of motile and morphologically normal stallion spermatozoa was attached to oviductal cell monolayers in vitro (Thomas et al 1994b). In addition, in humans, a higher proportion of morphologically normal spermatozoa were recovered from oviduct, bursa or peritoneum compared to spermatozoa found in caudal portions of the female reproductive system (Asch 1976, Mortimer et al 1982). Electron microscopy studies confirmed that a high percentage of sperm attached to oviductal epithelium had intact acrosomes (Esponda and Moreno 1998, Pollard et al 1991). Sperm morphology and motility are not the only criteria for oviductal sperm selection. Ellington et al (1999) have studied the chromatin quality of human sperm attached to bovine oviductal cells using the flow cytometric sperm chromatin structure assay (SCSA) (Evenson and Jost 1994). It has been demonstrated that human sperm bound to oviductal cells had higher chromatin quality than unbound sperm (Ellington et al 1999a). In conjunction, these data suggest that the oviduct isthmus functions as a screen for selecting a sperm subpopulation for fertilization.
SPERM FERTILIZING ABILITY. Perhaps the basic function of sperm storage is to ensure a successful meeting of male and female gametes in the oviduct by maintaining sperm viability and fertilizing ability (Pollard et al 1991). Establishment of this reservoir seems to be an important condition for successful fertilization. In the mare and other females with long estrus, a prolonged oviductal storage of sperm is required because fertilization can occur several days after mating (Day 1942). In these species, the ability of the oviductal environment to prolong fertility of sperm is of paramount importance to optimize the reproductive process. Extended sperm longevity in the oviduct may also be advantageous in species which either ovulate a large number of follicles over time or ovulate after estrus. Unlike other farm animals, cattle ovulate around 12 h after the end of the estrus; therefore, sperm are in the female's reproductive tract about 12-30 h before ovulation.
Sperm capacitation and active motility along with an intact acrosome are necessary conditions for successful fertilization. There is evidence that soluble products secreted by oviductal cells and/or direct contact of sperm with oviductal cells can extend sperm fertilizing capacity (Pollard et al 1991). In an attempt to mimic in vivo conditions, in vitro fertilization systems with sperm cocultured with OEC have been used (Chian and Sirard 1995; Pollard et al 1991). One of the main drawbacks of these systems is that it is not possible to know the real number of sperm available for fertilization. Chian and Sirard (1995) reported that the number of penetrated oocytes was similar in the coculture system compared to a standard system in which OEC were not present. Unfortunately, in this study the developmental capacity of embryos produced under these conditions was not determined. Further research to study developmental competence of embryos produced in this type of in vitro fertilization systems is needed.
MANIPULATION OF THE SPERM RESERVOIR TO IMPROVE REPRODUCTIVE PERFORMANCE
Considering the central role of the oviduct in gamete transport, fertilization and embryo growth (for review see: Ellington, 1991), it seems plausible that development of methods to control oviductal physiology would be of great value for improving reproductive performance in farm animals. Rabbit and sheep oviducts have been successfully used to grow preimplantation bovine embryos produced in vitro from the 1- to 2-cell stage up to the morula/blastocyst stage (Behboodi et al 1993, Ellington et al 1990, Fukui et al 1983, Leibfried-Rutledge et al 1987, Trounson et al 1977). Moreover, as reviewed by Bavister (1988), many laboratories have reported a beneficial effect of OEC in culture on in vitro fertilization (IVF) and in vitro embryo development.
Most of our knowledge about OEC-sperm interactions comes from studies in which sperm cells were cocultured with oviductal cells in vitro (Chian et al 1995, Chian and Sirard 1995, Dobrinski et al 1996b, Ellington et al 1998a, Ellington et al 1991, Lefebvre et al 1995, Suárez et al 1990). These studies have rendered invaluable information about the molecular basis of sperm-OEC attachment and sperm physiology in coculture with OEC. However, it should be pointed out that OEC cannot thoroughly mimic in vivo oviductal conditions (Walter, 1995).
Although our understanding of the molecular basis of sperm-oviductal cell interaction has increased considerably in recent years (Lefebvre et al 1997, Suárez 1998, Suárez et al 1998), the mechanisms that govern progression of sperm through the oviduct are still poorly understood. Evidence strongly suggests that ovarian steroids play a key role in controlling sperm migration to the site of fertilization in pigs (Hunter et al 1999, Hunter 1972, 1995, 1984, 1981). On the other hand, in cattle it has not been possible to establish a clear relationship between sperm build up in the isthmus and the stage of the estrous cycle and hormonal concentration (Lefebvre et al 1995). The number of bull sperm attached to oviduct explants was neither dependent on the region of oviduct (i.e., isthmus vs ampulla) nor on the stage of the estrous cycle (Lefebvre et al 1995).
Artificial insemination (AI) is a technique extensively used not only in dairy cattle but also in the beef industry. Since standing estrus in cattle is short (15-18 h) and insemination takes place approximately 12 h after estrus detection in the popular AM-PM breeding strategy, a proportion of females is inseminated at the early metestrus. Furthermore, spermatozoa are deposited into the uterus contrasting with vaginal deposition during natural mating. Therefore, under these AI conditions, the mechanisms that control the number of spermatozoa passing from the uterus to the site of fertilization in the oviduct may be less effective or somehow affected. Increased patency of the oviductal lumen after estrus is associated with a drop of estrogen plasmatic concentrations and, probably, increases of progesterone concentrations. In addition, the ability of sperm to attach to oviductal cells could be altered by molecular changes at the sperm surface caused by the freezing-thawing process (Ellington et al 1999b) or changes in the number of sperm binding sites on oviductal epithelial cells. An augmented sperm transport may frequently be the case when the sperm suspension is deposited into the uterus during metestrus. Accordingly, the risk of polyspermic fertilization is increased under these circumstances, and can negatively affect the conception rate and the profitability of the cattle industry. Understanding the mechanisms that regulate sperm entrapment and release from oviductal sperm storage is essential to development of strategies (e.g., hormonal treatments) in order to manipulate sperm transport through the female tract to maximize reproductive efficiency under different management conditions. Of particular interest is the development of methods to improve artificial insemination with low number of spermatozoa to maximize the use of ejaculates of valuable males and sexed semen.
The success of in vitro embryo production programs depends almost entirely on the quality of biological material used, e.g. oocytes and sperm. The number of transferable embryos produced in these programs depends on both high quality mature oocytes and adequate numbers of good quality motile spermatozoa. Since males ejaculate a heterogeneous population of sperm, several separation techniques such as swim up and density gradients (e.g., Percoll) have been developed (Bavister 1990, Mortimer 1990). These techniques not only allow for selection of sperm with enhanced motility but may also be used to remove the extender and dead cells (up 50% of total) present in frozen and thawed sperm samples (Bavister, 1990). Potential disadvantages of these techniques are low sperm recovery and risk of both mechanical and chemical damage to sperm (Bavister 1990). Based on the fact that OEC selectively binds a sperm subpopulation with low chromatin damage (Ellington et al 1999a) and low morphological abnormalities (Thomas et al 1994b), coculture could thus be used to select the best sperm for IVF (Gualtieri and Talevi 2003) and other assisted reproductive techniques including intracytoplasmic sperm injection.
REFERENCES
Abe H, Y Sendai, T Satoh, H Hoshi. 1995. Secretory products of bovine oviductal epithelial cells support the viability and motility of bovine spermatozoa in culture in vitro. J Exp Zool 272: 54-61.
Asch RH. 1976. Laparoscopic recovery of human sperm from peritoneal fluid, in patients with negative or poor sims-hunher test. Fertil Steril 27: 1111-1114.
Bavister BD. 1988. Role of oviductal secretions in embryonic growth in vivo and in vitro. Theriogenology 29, 143-154.
Bavister BD. 1990. Tests of sperm fertilizing ability. In: R. H. Asch, J. P. Balmaceda and I. Johnston (eds.) Gamete physiology. p 77-105. Serono Symposia, Nowell, Massachusetts.
Bedford JM. 1970. Sperm capacitation and fertilization in mammals. Biol Reprod Suppl 2, 128-158.
Behboodi E, GB Anderson, S Horvat, JF Medrano, JD Murray, JD Rowe. 1993. Microinjection of bovine embryos with a foreign gene and its detection at the blastocyst stage. J Dairy Sci 76, 3392-3399.
Boquest AC, JF Smith, RM Briggs, DM Duganzich, PM Summers. 1999. Effects of bovine oviductal proteins on bull spermatozoal function. Theriogenology 51, 583-595.
Boquest AC, PM Summers. 1999. Effects of 17ß-oestradiol or oestrous stage-specific cow serum on the ability of bovine oviductal epithelial cell monolayers to prolong the viability of bull spermatozoa. Anim Reprod Sci 57, 1-14.
Borland RM, S Hazra, JD Biggers, CP Lechene. 1977. The elemental composition of the environments of the gametes and preimplantation embryo during the initiation of pregnancy. Biol Reprod 16, 147-157.
Bosch P, JM De Avila, JE Ellington, RW Wright, Jr. 2001. Heparin and Ca2+-free medium can enhance release of bull sperm attached to oviductal epithelial cell monolayers. Theriogenology 56, 247-260.
Boyle MS, DG Cran, WR Allen, RH Hunter. 1987. Distribution of spermatozoa in the mare's oviduct. J Reprod Fertil Suppl. 35, 79-86.
Brundin J. 1965. Distribution and function of adrenergic nerves in the rabbit fallopian tube. Acta Physiol Scand Suppl 259, 1-57.
Burkman LJ, JW Overstreet, DF Katz. 1984. A possible role for potassium and pyruvate in the modulation of sperm motility in the rabbit oviductal isthmus. J Reprod Fertil 71, 367-376.
Chian RC, S Lapointe, MA Sirard. 1995. Capacitation in vitro of bovine spermatozoa by oviduct epithelial cell monolayer conditioned medium. Mol Reprod Dev 42, 318-324.
Chian RC, MA Sirard. 1995. Fertilizing ability of bovine spermatozoa cocultured with oviduct epithelial cells. Biol Reprod 52: 156-162.
Dasgupta S, CL Mills, LR Fraser. 1993. Ca2+-related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. J Reprod Fertil 99, 135-143.
David A, A Vilensky, H Nathan. 1971. Temperature changes in different parts of the rabbit oviduct. Preliminary report. Harefuah 80, 180-182.
Day BN, C Polge. 1968. Effect of progesterone on fertilization and egg transport in the pig. J Reprod Fertil 17, 227-230.
Day FT. 1942. Survival of spermatozoa in the genital tract of the mare. J Agric Sci 32, 108-111.
Demott RP, R Lefebvre, SS Suárez. 1995. Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod 52, 1395-1403.
Dobrinski I, GG Ignotz, GA Thomas, BA Ball. 1996a. Role of carbohydrates in the attachment of equine spermatozoa to uterine tubal (oviductal) epithelial cells in vitro. Am J Vet Res 57, 1635-1639.
Dobrinski, I., TT Smith, SS Suárez, BA Ball. 1997. Membrane contact with oviductal epithelium modulates the intracellular calcium concentration of equine spermatozoa in vitro. Biol Reprod 56, 861-869.
Dobrinski I, SS Suárez, BA Ball. 1996b. Intracellular calcium concentration in equine spermatozoa attached to oviductal epithelial cells in vitro. Biol Reprod 54, 783-788.
El-Banna AA, ES Hafez. 1970. Profile analysis of the oviductal wall in rabbits and cattle. Anat Rec 166, 469-478.
Ellington JE 1991. The bovine oviduct and its role in reproduction: A review of the literature. Cornell Vet 81, 313-328.
Ellington JE, BA Ball, BJ Blue, CE Wilker. 1993a. Capacitation-like membrane changes and prolonged viability in vitro of equine spermatozoa cultured with uterine tube epithelial cells. Am. J Vet Res 54, 1505-1510.
Ellington JE, BA Ball, X Yang. 1993b. Binding of stallion spermatozoa to the equine zona pellucida after coculture with oviductal epithelial cells. J Reprod Fertil 98, 203-208.
Ellington JE, DP Evenson, JE Fleming, RS Brisbois, GA Hiss, SJ Broder, RW Wright. 1998a. Coculture of human sperm with bovine oviduct epithelial cells decreases sperm chromatin structural changes seen during coculture in media alone. Fertil Steril 69, 643-649.
Ellington JE, DP Evenson, RW Wright, AE Jones, CS Schneider, GA Hiss, RS Brisbois. 1999a. Higher-quality human sperm in a sample selectively attach to oviduct (fallopian tube) epithelial cells in vitro. Fertil Steril 71, 924-929.
Ellington JE, PB Farrell, ME Simkin, RH Foote, EE Goldman, AB Mcgrath. 1990. Development and survival after transfer of cow embryos cultured from 1- 2-cells to morulae or blastocysts in rabbit oviducts or in a simple medium with bovine oviduct epithelial cells. J Reprod Fertil 89, 293-299.
Ellington JE, AE Jones, CM Davitt, CS Schneider, RS Brisbois, GA Hiss, RW Wright, Jr. 1998b. Human sperm function in co-culture with human, macaque or bovine oviduct epithelial cell monolayers. Hum Reprod 13, 2797-2804.
Ellington JE, AW Padilla, WL Vredenburgh, EP Dougherty, RH Foot. 1991. Behavior of bull spermatozoa in bovine uterine tube epithelial cell co-culture: An in vitro model for studying the cell interactions of reproduction. Theriogenology 35, 977-595.
Ellington JE, JC Samper, AE Jones, SA Oliver, KM Burnett, RW Wright. 1999b. In vitro interactions of cryopreserved stallion spermatozoa and oviduct (uterine tube) epithelial cells or their secretory products. Anim Reprod Sci 56, 51-65.
Esponda P, M Moreno. 1998. Acrosomal status of mouse spermatozoa in the oviductal isthmus. J Exp Zool 282, 360-366.
Evenson D, L Jost. 1994. Sperm chromatin structure assay: DNA denaturability. Methods Cell Biol 42, 159-176.
Fazeli A, EA Duncan, PF Watson, WV Holt. 1999. Sperm-oviduct interaction: Induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol Reprod 60, 879-886.
Fukui Y, M Fukushima, H Ono. 1983. Fertilization and cleavage of bovine follicular oocytes in rabbit reproductive tracts after maturation in vitro. J Exp Zool 226, 137-142.
Ginther OJ. 1974. Internal regulation of physiological processes through local venoarterial pathways: A review. J Anim Sci 39, 550-564.
Ginther OJ. 1976. Comparative anatomy of utero-ovarian vasculature. Veterinary Scope 20, 1-17.
Gualtieri R, R Talevi. 2000. In vitro-cultured bovine oviductal cells bind acrosome-intact sperm and retain this ability upon sperm release. Biol Reprod 62, 1754-1762.
Gualtieri R, R Talevi. 2003. Selection of highly fertilization-competent bovine spermatozoa through adhesion to the fallopian tube epithelium in vitro. Reproduction 125, 251-258.
Gutiérrez A, J Garde, C García-Artiga, I Vázquez. 1993. Ram spermatozoa cocultured with epithelial cell monolayers: An in vitro model for the study of capacitation and acrosome reaction. Mol Reprod Dev 36, 338-345.
Guyader C, D Chupin. 1991. Capacitation of fresh bovine spermatozoa on bovine epithelial oviduct cell monolayers. Theriogenology 36, 505-512.
Gwathmey TM, GG Ignotz, SS Suárez. 2003. Pdc-109 (bsp-a1/a2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol Reprod 69, 809-815.
Hunter RH, B Flechon, JE Flechon. 1991. Distribution, morphology and epithelial interactions of bovine spermatozoa in the oviduct before and after ovulation: A scanning electron microscope study. Tissue Cell 23, 641-656.
Hunter RH, R Nichol. 1986. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J Reprod Fertil 77, 599-606.
Hunter RH, HH Petersen, T Greve. 1999. Ovarian follicular fluid, progesterone and Ca2+ ion influences on sperm release from the fallopian tube reservoir. Mol Reprod Dev 54, 283-291.
Hunter RHF. 1972. Local action of progesterone leading to polyspermic fertilization in pigs. J Reprod Fertil 31, 433-444.
Hunter RHF. 1980. Physiology and technology of reproduction in female domestic animals. Academic Press Inc., London.
Hunter RHF. 1981. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J Reprod Fertil 63, 109-117.
Hunter RHF. 1984. Pre-ovulatory arrest and peri-ovulatory distribution of competent spermatozoa in the isthmus of the pig oviduct. J Reprod Fertil 72, 203-211.
Hunter RHF. 1987. The timing of capacitation in mammalian spermatozoa-a reinterpretation. Res Reprod 19, 3-4.
Hunter RHF. 1995. Ovarian endocrine control of sperm progression in the fallopian tubes. Oxford. Rev Reprod Biol 17, 85-124.Hunter RHF. 1996. Ovarian control of very low sperm/egg ratios at the commencement of mammalian fertilisation to avoid polyspermy. Mol Reprod Dev 44, 417-422.
Ignotz GG, MC Lo, CL Pérez, TM Gwathmey, SS Suárez. 2001. Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol Reprod 64, 1806-1811.
Jansen RP. 1978. Fallopian tube isthmic mucus and ovum transport. Science 201, 349-351.
Jansen RP. 1984. Endocrine response in the fallopian tube. Endocr Rev 5, 525-551.
Jansen RP, VK Bajpai. 1982. Oviduct acid mucus glycoproteins in the estrous rabbit: Ultrastructure and histochemistry. Biol Reprod 26, 155-168.
Joshi MS. 1988. Isolation, cell culture and immunocytochemical characterization of oviduct epithelial cells of the cow. J Reprod Fertil 83, 249-261.
Joshi MS. 1991. Growth and differentiation of the cultured secretory cells of the cow oviduct on reconstituted basement membrane. J Exp Zool 260, 229-238.
Kervancioglu ME O Djahanbakhch, RJ Aitken. 1994. Epithelial cell coculture and the induction of sperm capacitation. Fertil Steril 61, 1103-1108.
Leese HJ. 1988. The formation and function of oviduct fluid. J Reprod Fertil 82, 843-856.
Lefebvre R, PJ Chenoweth, M Drost, CL Leclear, M Maccubbin, JT Dutton, SS Suárez. 1995. Characterization of the oviductal sperm reservoir in cattle. Biol Reprod 53, 1066-1074.
Lefebvre R, MC Lo, SS Suárez. 1997. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol. Reprod. 56, 1198-1204.
Lefebvre R, SS Suárez. 1996. Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol Reprod 54, 575-582.
Leibfried-Rutledge ML, ES Critser, WH Eyestone, DL Northey, NL First. 1987. Development potential of bovine oocytes matured in vitro or in vivo. Biol Reprod 36, 376-383.
McCracken JA, W Schramm, N Einer-Jensen. 1984. The structure of steroids and their diffusion through blood vessel walls in a counter-current system. Steroids 43, 293-303.
McDaniel JW, H Scalzi, DL Black. 1968. Influence of ovarian hormones on histology and histochemistry of the bovine oviduct. J Dairy Sci 51, 754-761.
Mortimer D. 1990. Sperm analysis and sperm washing techniques. In: C. Gagnon (ed.) Controls of sperm motility: Biological and clinical aspects. p 263-284. CRC Press, Boca Raton, Florida.
Mortimer D, EE Leslie, RW Kelly, AA Templeton. 1982. Morphological selection of human spermatozoa in vivo and in vitro. J Reprod Fertil 64, 391-399.
Murdoch RN, IG White. 1971. Studies of the stimulating effect of bicarbonate on the metabolism of ram spermatozoa. J Reprod Fertil 25, 231-242.
Nichol R, RH Hunter, DK Gardner, HJ Leese, GM Cooke. 1992. Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period. J Reprod Fertil 96, 699-707.
Overstreet JW, GW Cooper. 1975. Reduced sperm motility in the isthmus of the rabbit oviduct. Nature 258, 718-719.
Overstreet JW, DF Katz, LL Johnson. 1980. Motility of rabbit spermatozoa in the secretions of the oviduct. Biol Reprod 22, 1083-1088.
Pacey AA, N Davies, MA Warren, CLR Barratt, ID Cooke. 1995a. Hyperactivation may assist human spermatozoa to detach from intimate association with the endosalpinx. Hum Reprod 10, 2603-2609.
Pacey AA, CJ Hill, IW Scudamore, MA Warren, CLR Barratt, ID Cooke. 1995b. The interaction in vitro of human spermatozoa with epithelial cells from the human uterine (fallopian) tube. Hum Reprod 10, 360-366.
Parrish JJ, JL Susko-Parrish, RR Handrow, MM Sims, NL First. 1989. Capacitation of bovine spermatozoa by oviduct fluid. Biol Reprod 40, 1020-1025.
Petrunkina AM, R Gehlhaar, W Drommer, D Waberski, E Topfer-Petersen. 2001. Selective sperm binding to pig oviductal epithelium in vitro. Reproduction 121, 889-896.
Pollard JW, C Plante, WA King, PJ Hansen, KJ Betteridge, SS Suárez. 1991. Fertilizing capacity of bovine sperm may be maintained by binding to oviductal epithelial cells. Biol Reprod 44, 102-107.
Raychoudhury SS, SS Suárez. 1991. Porcine sperm binding to oviductal explants in culture. Theriogenology 36, 1059-1070.
Revah I, BM Gadella, FM Flesch, B Colenbrander, SS Suárez. 2000. Physiological state of bull sperm affects fucose- and mannose-binding properties. Biol Reprod 62, 1010-1015.
Rodríguez-Martínez H, P Tienthai, K Suzuki, H Funahashi, H Ekwall, A Johannisson. 2001. Involvement of oviduct in sperm capacitation and oocyte development in pigs. Reprod Suppl 58, 129-145.
Schramm W, N Einer-Jensen, G Schramm, JA Mccracken. 1986. Local exchange of oxytocin from the ovarian vein to ovarian arteries in sheep. Biol Reprod 34, 671-680.
Smith TT. 1998. The modulation of sperm function by the oviductal epithelium. Biol Reprod 58, 1102-1104.
Smith TT, WB Nothnick. 1997. Role of direct contact between spermatozoa and oviductal epithelial cells in maintaining rabbit sperm viability. Biol Reprod 56, 83-89.
Smith TT, R Yanagimachi. 1990. The viability of hamster spermatozoa stored in the isthmus of the oviduct: The importance of sperm-epithelium contact for sperm survival. Biol Reprod 42, 450-457.
Smith TT, R Yanagimachi. 1991. Attachment and release of spermatozoa from the caudal isthmus of the hamster oviduct. J Reprod Fertil 91, 567-573.
Suárez S, K Redfern, P Raynor, F Martin, DM Phillips. 1991. Attachment of boar sperm to mucosal explants of oviduct in vitro, Possible role in formation of a sperm reservoir. Biol Reprod 44, 998-1004.
Suárez SS. 1987. Sperm transport and motility in the mouse oviduct: Observations in situ. Biol Reprod 36, 203-210.
Suárez SS. 1998. The oviductal sperm reservoir in mammals: Mechanisms of formation. Biol Reprod 58, 1105-1107.
Suárez SS. 2002. Formation of a reservoir of sperm in the oviduct. Reprod Domest Anim 37, 140-143.Suárez SS, K Brockman, R Lefebvre. 1997. Distribution of mucus and sperm in bovine oviduct after artificial insemination: The physical environment of the oviductal sperm reservoir. Biol Reprod 56, 447-453.
Suárez SS, M Drost, K Redfern, W Gottlleb. 1990. Sperm motility in the oviduct. In: B. D. Bavister, J. Cummins and E. R. S. Roldan (eds.) Fertilization in mammals. p 111-124. Serono Symposia, Norwell, Massachusetts.
Suárez SS, I Revah, M Lo, S Kölle. 1998. Bull sperm binding to oviductal epithelium is mediated by Ca2+-dependent lectin on sperm that recognizes lewis-a trisaccharide. Biol Reprod 59, 39-44.
Suzuki K, A Asano, B Eriksson, K Niwa, T Nagai, H Rodríguez-Martínez. 2002. Capacitation status and in vitro fertility of boar spermatozoa: Effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int J Androl 25, 84-93.
Suzuki K, B Eriksson, H Shimizu, T Nagai, H Rodríguez-Martínez. 2000. Effect of hyaluronan on monospermic penetration of porcine oocytes fertilized in vitro. Int J Androl 23, 13-21.
Talevi R, R Gualtieri. 2001. Sulfated glycoconjugates are powerful modulators of bovine sperm adhesion and release from the oviductal epithelium in vitro. Biol Reprod 64, 491-498.
Thomas PGA, BA Ball. 1996. Cytofluorescent assay to quantify adhesion of equine spermatozoa to oviduct epithelial cells in vitro. Mol Reprod Dev 43, 55-61.
Thomas PGA, BA Ball, SP Brinsko. 1994a. Interaction of equine spermatozoa with oviductal epithelial cell explants is affected by estrous cycle and anatomic origin of explant. Biol Reprod 51, 222-228.
Thomas PGA, BA Ball, PG Miller, SP Brinsko, L Southwood. 1994b. A subpopulation of morphologically normal, motile spermatozoa attach to equine oviductal epithelial cell monolayers. Biol Reprod 51, 303-309.
Topfer-Petersen E, A Wagner, J Friedrich, A Petrunkina, M Ekhlasi-Hundrieser, D Waberski, W Drommer. 2002. Function of the mammalian oviductal sperm reservoir. J. Exp. Zool. 292, 210-215.
Trounson AO, SM Willadsen, LE Rowson. 1977. Fertilization and development capability of bovine follicular oocytes matured in vitro and in vivo and transferred to the oviducts of rabbits and cows. J Reprod Fertil 51, 321-327.
Walter I. 1995. Culture of bovine oviduct epithelial cells (boec). Anat Rec 243, 347-356.
Wilmut I, HF Hunter. 1984. Sperm transport into the oviducts of heifers mated early in oestrus. Reprod Nutr Dev 24, 461-468.
Yanagimachi R. 1990. Capacitation and acrosome reaction. In: R. H. Asch, J. P. Balmaceda and I. Johnston (eds.) Gamete Physiology. p 31-42. Serono Symposia, Nowell, Massachusetts.
Aceptado: 14.12.04.
* Present address: Department of Animal and Dairy Science, The University of Georgia, Athens GA 30602.